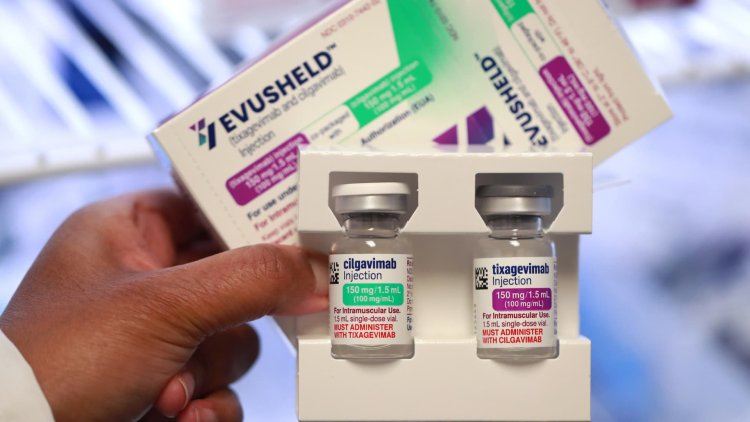
FDA withdraws Covid antibody treatment Evusheld because it's not effective against 93% of subvariants
Many take Evusheld as an additional layer of protection because the vaccines do not trigger a strong immune response for them.
Join our subscribers list to get the latest news, updates and special offers directly in your inbox








LocalNews Nov 16, 2022 0 10
LocalNews Oct 24, 2022 0 4
LocalNews Apr 29, 2023 0 180
The challenge of recruiting and retaining talent in the post-Covid world continues...
LocalNews Apr 27, 2023 0 51
The ruling will reverberate worldwide
LocalNews Apr 23, 2023 0 55
Dominic Raab’s exit from Britain’s government, and the role of fear in the workplace
submissions@upscene.com Jan 26, 2023 0 255
Database Workbench 6 is the next major version of the popular database development...
The perfect portable power station solution provider for personal and enterprises Sep 24, 2022 0 36
With Ryangi energy, you have all the charging options you need in one compact, reliable...